FDA End Use Letter Template for Regulatory Compliance

htmlEdit
When engaging in international trade or transferring goods that require regulatory oversight, certain formal documents play a crucial role in ensuring proper usage and adherence to legal standards. These documents serve as a means of confirming the intended destination and application of the products, ensuring that they comply with relevant regulations and restrictions.
Understanding the structure and purpose of such a document is essential for businesses and individuals who navigate global markets. It provides clarity on the compliance requirements and serves as a protective measure against potential legal challenges. By clearly stating the purpose and final location of the goods, this document helps in obtaining necessary approvals and facilitating smooth transactions.
Crafting an effective document involves understanding the key elements that need to be addressed, including the specific details of the product, the recipient, and the anticipated end goal. This ensures transparency and maintains the integrity of the compliance process.
htmlEdit
Understanding the Regulatory Compliance Document

In the context of international trade, certain formal documents are essential for confirming that goods will be used in a manner consistent with applicable laws and regulations. These documents are key to verifying the ultimate application and destination of products, ensuring that they do not violate any restrictions or intended purposes set by governing bodies.
The structure of such a document typically includes details about the product, its intended recipient, and the overall purpose for which it is being transferred. This documentation serves as a means to assure authorities that the goods will not be misused or diverted to unauthorized markets.
By understanding the significance of this compliance tool, organizations can ensure smooth transactions and avoid potential legal issues. Properly completed documents are critical in obtaining the necessary permissions and ensuring adherence to regulatory requirements throughout the product’s lifecycle.
htmlEdit
What is a Compliance Declaration?
A compliance declaration is a formal document used to confirm the intended application and destination of specific goods or materials being exported or imported. This written statement serves as a means to assure regulatory authorities that the products in question will be used in accordance with the relevant laws and regulations. It is often required when goods are subject to specific restrictions or oversight due to safety, security, or trade compliance concerns.
Purpose and function of this document is to provide transparency in the movement of goods, ensuring that they are not diverted to unauthorized uses. It typically includes information about the product, its final destination, and how it will be applied in the recipient’s operations. This declaration is crucial for industries involved in cross-border trade, as it helps in obtaining the necessary approvals for shipment and ensures that all legal obligations are met.
Crafting an effective compliance statement requires attention to detail, as it must accurately describe the intended use and recipient of the goods. The information provided must be clear and verifiable to facilitate the approval process and avoid delays or legal complications.
htmlEdit
Why is it Important for Compliance?
Ensuring that products are used in accordance with established regulations is crucial for businesses involved in international trade. Compliance documentation plays a vital role in confirming that goods are not misdirected or misused, which could result in legal issues or penalties. This documentation serves as an essential tool for businesses to demonstrate adherence to laws governing safety, trade, and security.
Regulatory adherence is necessary for avoiding disruptions in operations, preventing financial penalties, and maintaining a company’s reputation. When companies fail to meet these requirements, they risk facing delays, customs issues, or even bans on further shipments. Such consequences can significantly impact a company’s operations, especially when exporting to regulated markets.
By providing clear, accurate information about the final destination and intended application of goods, compliance documentation ensures that all parties involved can verify the proper handling of products. This not only guarantees legal compliance but also fosters trust between businesses, authorities, and consumers.
htmlEdit
Key Components of a Compliance Declaration
A well-crafted compliance declaration includes several crucial elements that ensure transparency and meet regulatory requirements. These key components provide authorities with all the necessary details to assess whether the goods will be used in accordance with relevant laws and restrictions. The document must be clear, accurate, and comprehensive to avoid delays or issues with shipments.
Product Information
First and foremost, the declaration should include detailed descriptions of the products being exported or imported. This typically covers the nature, quantity, and specifications of the goods. Accurate product details help verify that the items match what is outlined in trade agreements and regulations.
Recipient and Intended Application
Another vital component is information about the recipient and how the goods will be utilized. This section should clarify who will receive the products and the intended purpose, ensuring the items are not diverted to unauthorized uses. Providing precise details regarding the final destination and end application is essential for compliance purposes.
htmlEdit
Common Mistakes to Avoid
When drafting a compliance document, attention to detail is crucial. Small errors can lead to delays in the approval process or even result in legal consequences. Understanding common pitfalls helps ensure that the document meets all regulatory requirements and facilitates smooth transactions. Below are some frequent mistakes to avoid when preparing such declarations.
Inaccurate Product Details

One of the most common mistakes is providing incomplete or inaccurate information about the products. This can include errors in product descriptions, quantities, or specifications. It’s essential to ensure that the information is precise and matches what is listed in shipping and customs records to avoid confusion or rejections from regulatory bodies.
Vague or Missing Intended Use Information
Another critical mistake is failing to clearly state how the goods will be used or where they will be delivered. A vague or ambiguous description of the recipient or the product’s end goal can raise red flags. Ensure that the document clearly outlines the intended application and destination of the items to prevent any misunderstanding or non-compliance issues.
htmlEdit
How to Draft an Effective Compliance Document
Creating a well-organized and clear compliance document is essential for ensuring that all regulatory requirements are met. A well-structured statement will not only provide necessary details but also help prevent potential delays or legal complications. Here are some steps to follow when drafting an effective document.
Ensure Accuracy and Clarity
The most important aspect of any compliance declaration is accuracy. Each section of the document should contain precise details about the goods, their destination, and their intended application. Avoid vague language and ensure that all information is easily verifiable. This minimizes the risk of misunderstandings and improves the chances of smooth approval from regulatory authorities.
Organize Information Logically
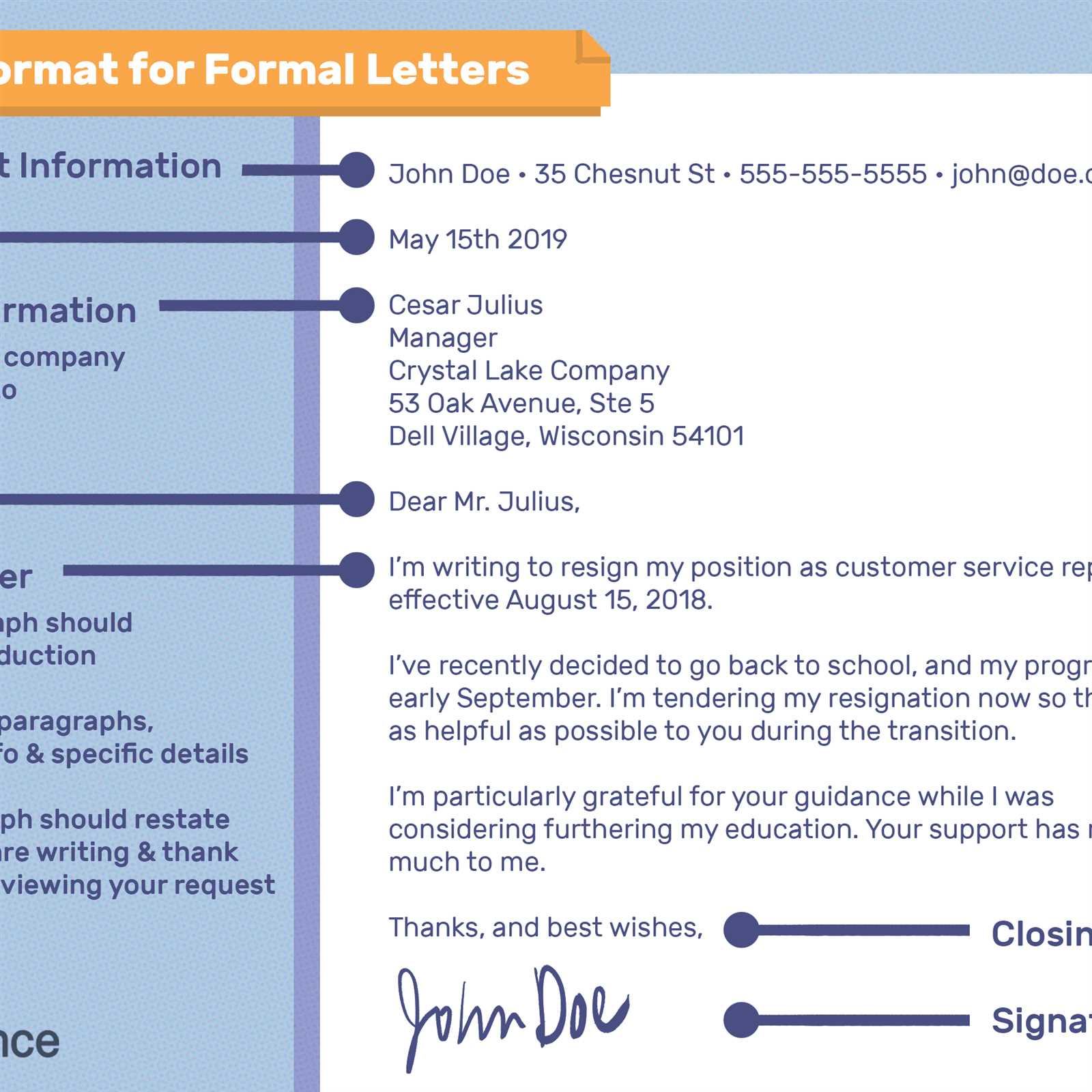
A well-organized document is easier to understand and more likely to be approved. Break down the information into clear sections that are easy to follow. Use headings and subheadings to separate different categories, such as product details, recipient information, and the intended purpose of the goods. Below is a simple example of how to structure the document:
| Section | Description |
|---|---|
| Product Details | Provide a clear description of the goods, including specifications and quantities. |
| Recipient Information | Include details about the recipient, such as name, address, and contact information. |
| Intended Application | Explain how the goods will be used and ensure there are no ambiguities about their purpose. |
| Regulatory Compliance | Ensure the document highlights adherence to relevant laws and regulations governing the products. |
htmlEdit
Impact on International Trade Regulations

Compliance with international trade regulations is a critical factor in the global movement of goods. Formal declarations play a significant role in ensuring that shipments adhere to the requirements set by authorities in various countries. The accuracy and clarity of these documents are vital in maintaining smooth trade operations and avoiding disruptions due to legal or regulatory violations.
Influence on Customs Processes
Properly drafted compliance declarations can expedite customs clearance, as they provide necessary information to border authorities. When the required details are included and verified, customs officers can quickly assess the legality of the goods and ensure that no restrictions are being violated. Failure to provide complete or accurate information may lead to:
- Delays in shipment processing
- Increased inspection and review times
- Potential fines or penalties
- Rejection of goods at the border
Ensuring Adherence to Trade Agreements

International trade is governed by a complex web of agreements between countries. Compliance documents ensure that the transfer of goods adheres to the terms of these agreements, which may include restrictions on certain products or conditions for their sale. These documents help maintain trust between trading partners and avoid potential conflicts that could arise from violations of trade regulations.
In addition, failure to meet compliance standards can affect a company’s ability to engage in future trade, as regulatory bodies may impose restrictions on non-compliant businesses, making it difficult to do business in certain markets.